DESCRIPTION
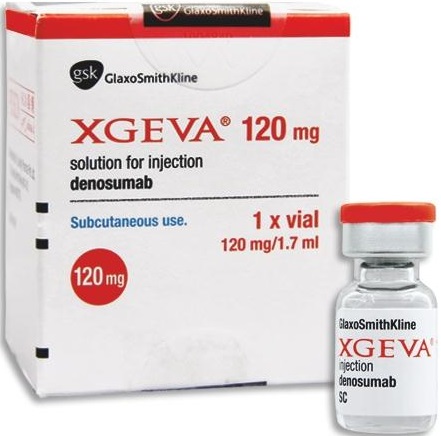
Xgeva 120mg (Denosumab) Injection is a prescription drug used in the treatment of osteoporosis and it is the only subcutaneous treatment option to prevent bone complications which usually occurs after receiving certain treatments that increase risk of fractures. It works by binding with a protein that causes bone loss, strengthens the bone and thereby minimizes the risk of fractures.
Manufacturer : Glaxo SmithKline Pharmaceuticals Ltd.
ADDITIONAL INFORMATION
Strengths available : Pack of 120mg / 1.7ml vial
Storage : Store at 2°C – 8°C
Dosage :
Xgeva is intended for subcutaneous route only and should not be administered intravenously, intramuscularly, or intradermally.
Multiple Myeloma and Bone Metastasis from Solid Tumors
The recommended dose of Xgeva is 120 mg administered as a subcutaneous injection every 4 weeks in the upper arm, upper thigh, or abdomen.
Giant Cell Tumor of Bone
The recommended dose of Xgeva is 120 mg administered every 4 weeks with additional 120 mg doses on Days 8 and 15 of the first month of therapy. Administer subcutaneously in the upper arm, upper thigh, or abdomen.
Administer calcium and vitamin D as necessary to treat or prevent hypocalcemia [see Warnings and Precautions (5.3)].
Hypercalcemia of Malignancy
The recommended dose of Xgeva is 120 mg administered every 4 weeks with additional 120 mg doses on Days 8 and 15 of the first month of therapy. Administer subcutaneously in the upper arm, upper thigh, or abdomen.
SIDE EFFECTS
Most common side effects are pain in extremity, Musculoskeletal (bone, muscle or joint) pain.
PACK SIZE
1 x 120mg vial per pack
3S Corporation is a WHO GDSP approved & licensed Pharmaceutical stockist/wholesaler/distributor/exporter/importer of XGEVA Denosumab 120mg / 1.7ml Injection based in India - To buy XGEVA Denosumab 120mg / 1.7ml Injection or know its cost price contact us here.
We supply & sell XGEVA Denosumab 120mg / 1.7ml Injection for Reference Listed Drugs, Government Tenders, Shortage Lists, Emergency Imports, Name Patient Drugs, Comparator Drug Studies, Un-licensed Importation, clinical trial samples & Bio-Similars.