Disclaimer
All trademarks, logos and brand names are the property of their respective owners, used here solely for identification & reference only under Section 30(1) of the Trade Marks Act, 1999. We are not affiliated with or endorsed by them unless explicitly stated. By using this website, you agree to these terms.
DESCRIPTION
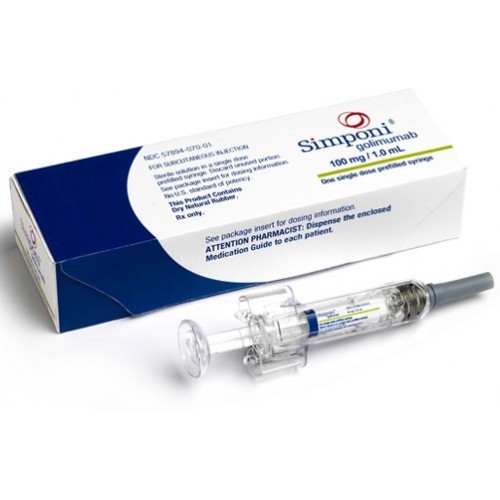
Simponi is a prescription drug used to treat ulcerative colitis, rheumatoid arthritis, psoriatic arthritis and ankylosing spondylitis. It is also used to reduce the effects of a substance in the body that can cause inflammation.To buy Simponi or to know its price contact 3S Corporation.
Manufacturer : Janssen Pharmaceuticals, Inc
ADDITIONAL INFORMATION
Strengths available : 50 mg and 100 mg
Form :injection
Dosage :Dosage In Rheumatoid Arthritis, Psoriatic Arthritis, Ankylosing Spondylitis :-The Simponi dose regimen is 50 mg administered by subcutaneous injection once a month.For patients with rheumatoid arthritis (RA), Simponi should be given in combination with methotrexate and for patients with psoriatic arthritis (PsA) or ankylosing spondylitis (AS), Simponi may be given with or without methotrexate or other nonbiologic Disease-Modifying Antirheumatic Drugs (DMARDs). For patients with RA, PsA, or AS, corticosteroids, non-biologic DMARDs, and/or NSAIDs may be continued during treatment with Simponi.
Dosage In Moderately To Severely Active Ulcerative Colitis :-The recommended Simponi induction dosage regimen is a 200-mg subcutaneous injection at Week 0, followed by 100 mg at Week 2, and then maintenance therapy with 100 mg every 4 weeks.
Storage :Store at 2 °C to 8 °C
Is the medicine FDA approved : Yes
Date of Approval :24 April, 2009
SIDE EFFECTS
Common side effects are cough, chills, body ache or pain, ear congestion, difficulty with breathing, headache, fever, muscle aches, loss of voice, sore throat, sneezing, unusual tiredness or weakness, stuffy or running nose.
PACK SIZE
Pack of 1 single dose
3S Corporation is a WHO GDSP approved & licensed Pharmaceutical stockist/wholesaler/distributor/exporter/importer of Simponi Golimumab 50 mg and 100 mg based in India - To buy Simponi Golimumab 50 mg and 100 mg or know its cost price contact us here.
We supply & sell Simponi Golimumab 50 mg and 100 mg for Reference Listed Drugs, Government Tenders, Shortage Lists, Emergency Imports, Name Patient Drugs, Comparator Drug Studies, Un-licensed Importation, clinical trial samples & Bio-Similars.