Disclaimer
All trademarks, logos and brand names are the property of their respective owners, used here solely for identification & reference only under Section 30(1) of the Trade Marks Act, 1999. We are not affiliated with or endorsed by them unless explicitly stated. By using this website, you agree to these terms.
DESCRIPTION
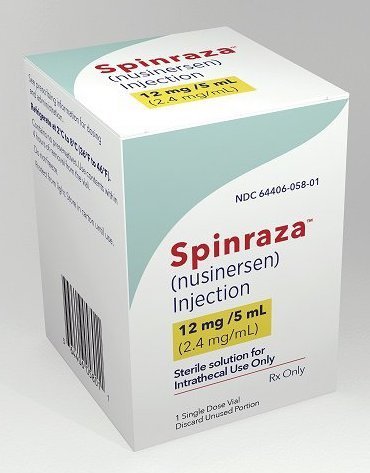
SPINRAZA (Nusinersen) is a prescription drug used to treat spinal muscular atrophy (SMA) in pediatric and adult patients. SMA is a rare genetic disease caused by a low level of protein in the motor neuron that are responsible for normal muscle functioning .The lack of protein causes motor neuron loss, progressive muscle weakness and may lead to premature death from respiratory failure. This is mostly observed among children and young adults. SPINRAZA increases the body’s ability to produce SMN protein critical to the health of motor neurons.
Manufacturer : Biogen Inc.
ADDITIONAL INFORMATION
Strengths available : 12mg / 5ml
Form : Injection
Storage : Store at temperature between 2°C – 8°C.
FDA Approval : USA Approved
Approval date: December 23, 2016
Dosage :
Initial: 4 loading doses; administer the first 3 doses at 14-day intervals and the fourth dose 30 days after the third dose
Maintenance: One dose every 4 months
SIDE EFFECTS
Common side effects of Spinraza include lower respiratory infection, upper respiratory infection, constipation, teething, congestion, ear infection and scoliosis.
PACK SIZE
Pack of 1 Vial
3S Corporation is a WHO GDSP approved & licensed Pharmaceutical stockist/wholesaler/distributor/exporter/importer of Spinraza Nusinersen 12mg/5ml Injection based in India - To buy Spinraza Nusinersen 12mg/5ml Injection or know its cost price contact us here.
We supply & sell Spinraza Nusinersen 12mg/5ml Injection for Reference Listed Drugs, Government Tenders, Shortage Lists, Emergency Imports, Name Patient Drugs, Comparator Drug Studies, Un-licensed Importation, clinical trial samples & Bio-Similars.