Description
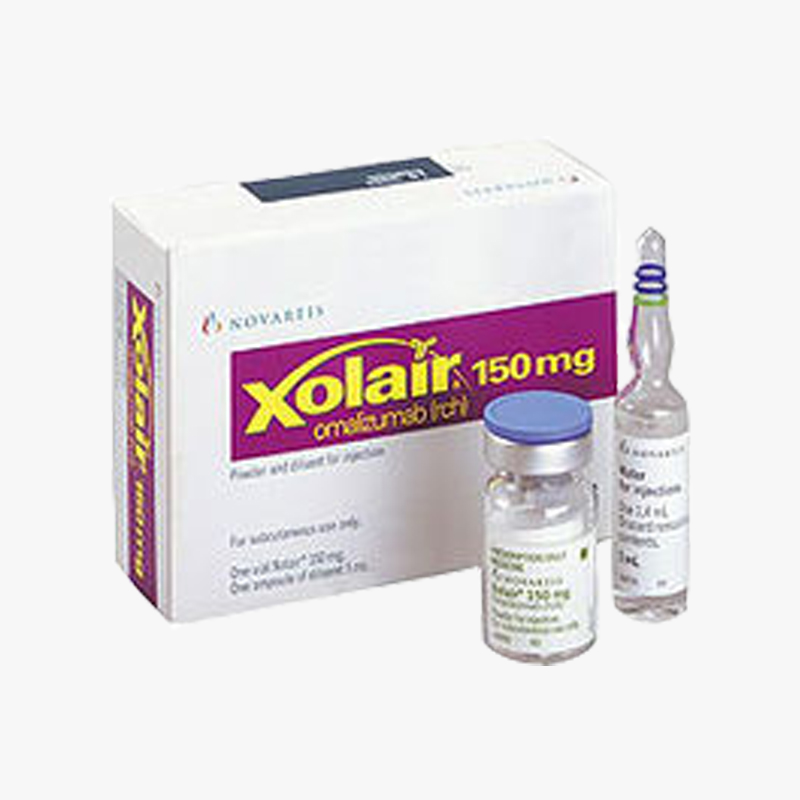
Xolair (Omalizumab)150 mg injection from Novartis is a prescription medicine for patients 12 years of age and older. It is for patients with moderate to severe persistent allergic asthma caused by year-round allergens in the air. A skin or blood test is done generally to check if you have allergic asthma. XOLAIR is for patients who are not controlled by asthma medicines called inhaled steroids. Xolair helps reduce the number of asthma attacks in people with allergic asthma who still have asthma symptoms even though they are taking inhaled steroids.
Important Limitations of Use :
Xolair has not been proven to work in other allergic conditions.
Xolair is not a rescue medicine and should not be used to treat sudden asthma attacks.
Xolair should not be used in children under 12 years of age.
Manufacturer: Novartis
Additional Information
Side Effects
Pack Size
3S Corporation is a WHO GDSP approved & licensed Pharmaceutical stockist/wholesaler/distributor/exporter/importer of Xolair 150 mg Injection based in India - To buy Xolair 150 mg Injection or know its cost price contact us here.
We supply & sell Xolair 150 mg Injection for Reference Listed Drugs, Government Tenders, Shortage Lists, Emergency Imports, Name Patient Drugs, Comparator Drug Studies, Un-licensed Importation, clinical trial samples & Bio-Similars.